
Calcium sulfate
Calcium sulfate is the inorganic compound with the formula CaSO4 and related hydrates. In the form of anhydrite (the anhydrous form), it is used as a desiccant. One particular hydrate is better known as plaster of Paris, and another occurs naturally as the mineral gypsum. It has many uses in industry. All forms are white solids that are poorly soluble in water. Calcium sulfate causes permanent hardness in water. The compound exists in three levels of hydration corresponding to different crystallographic structures and to minerals.
- CaSO4 (anhydrite): anhydrous state
- CaSO4·2H2O (gypsum): dihydrate
- CaSO4 1/2H2O (bassanite): hemihydrate, also known as plaster of Paris. Specific hemihydrates are sometimes distinguished: α-hemihydrate and β-hemihydrate.
The main use of calcium sulfate is to produce plaster of Paris and stucco. These applications exploit the fact that calcium sulfate which has been powdered and calcined forms a moldable paste upon hydration. and hardens as crystalline calcium sulfate dihydrate. It is also convenient that calcium sulfate is poorly soluble in water. and does not readily dissolve in contact with water after its solidification.
The dissolution of the different crystalline phases of calcium sulfate in water is exothermic and releases heat. (decrease in Enthalpy: ΔH < 0). As an immediate consequence, to proceed, the dissolution reaction needs to evacuate this heat. that can be considered as a product of reaction. If the system is cooled, the dissolution equilibrium will evolve towards the right according to the Le Chatelier principle. and calcium sulfate will dissolve more easily.
Thus the solubility of calcium sulfate increases as the temperature decreases and vice versa. If the temperature of the system is raised, the reaction heat cannot dissipate. and the equilibrium will regress towards the left according to Le Chatelier principle. The solubility of calcium sulfate decreases as temperature increases. This counter-intuitive solubility behaviour is called retrograde solubility. It is less common than for most of the salts. whose dissolution reaction is endothermic (i.e, the reaction consumes heat: increase in Enthalpy: ΔH > 0. ) and whose solubility increases with temperature.
Another calcium compound, calcium hydroxide (Ca(OH)2, portlandite) also exhibits a retrograde solubility for the same thermodynamic reason. because its dissolution reaction is also exothermic and releases heat. So, to dissolve the maximum amount of calcium sulfate or the calcium hydroxide in water. it is necessary to cool the solution down close to its freezing point instead of increasing its temperature.
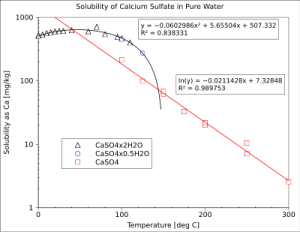
Temperature dependence of the solubility of calcium sulfate (3 phases) in pure water.
Hydration and dehydration reactions
With judicious heating, the gypsum converts to the partially dehydrated mineral called bassanite or plaster of Paris. This material has the formula CaSO4· (nH2O), where 0.5 ≤ n ≤ 0.8. Temperatures between 100 and 150 °C (212–302 °F) are required to drive off the water within its structure. The details of the temperature and the time depend on ambient humidity.
Temperatures as high as 170 °C (338 °F) are used in industrial calcination. but at these temperatures γ-anhydrite begins to form. The heat energy delivered to gypsum at this time (hydration heat) tends to go into driving off water(as water vapor. ) rather than increasing the temperature of the mineral, which rises slowly until the water is gone, then increases more rapidly. The equation for the partial dehydration is.
CaSO4 2 H2O → CaSO4 1/2 H2O + 1+1/2 H2O↑.
The endotermic property of this reaction is relevant to performance of drywall, conferring fire resistance to residential and other structures. In a fire, the structure behind a sheet of drywall will remain relatively cool as water is lost from gypsum. thus preventing (or substantially retarding) damage to framing (through wood combustion members or strength loss of steel at high temperatures) and consequent structural collapse.
But at higher temperatures, calcium sulfate will release oxygen and act as an oxidizing agent. This property is used in aluminothermy. contrast to most minerals, when rehydrated simply form liquid or semi-liquid pastes, or remain powdery, calcined gypsum has unusual property. when mixed with water at normal (ambient) temperatures, it quickly reverts chemically to the preferred dihydrate form. while physically “setting” to form a rigid and relatively strong gypsum crystal lattice. CaSO4 1/2 H2O + 1+1/2H2O → CaSO4 2 H2O.
This reaction is exothermic and is responsible for the ease with which gypsum can be cast into various shapes. including sheets (for drywall), sticks (for blackboard chalk), and molds (to immobilize broken bones, or for metal casting). Mixed with polymers, it has been used as a bone repair cement. Small amounts of calcined gypsum are added to earth to create strong structures directly from cast earth. an alternative to adobe (which loses its strength when wet).
The conditions of dehydration can be changed to adjust the porosity of the hemihydrate. resulting in the so-called α- and β-hemihydrates (which are more or less chemically identical) On heating to 180 °C (356 °F), the nearly water-free form, called γ-anhydrite (CaSO4·nH2O, n = 0to 0.05) is produced. γ-Anhydrite reacts slowly with water to return to the dihydrate state, a property exploited in some commercial desiccants.
On heating above 250 °C, the completely anhydrous form called β-anhydrite or “natural” anhydrite is formed. Natural anhydrite does not react with water, even over geological timescales, unless very finely ground. variable composition of hemihydrate and γ-anhydrite, and their easy inter-conversion, is due to their nearly identical crystal structures containing “channels. ” that can accommodate variable amounts of water, or other small molecules such as methanol.







