
Calcium Sulfate Forms
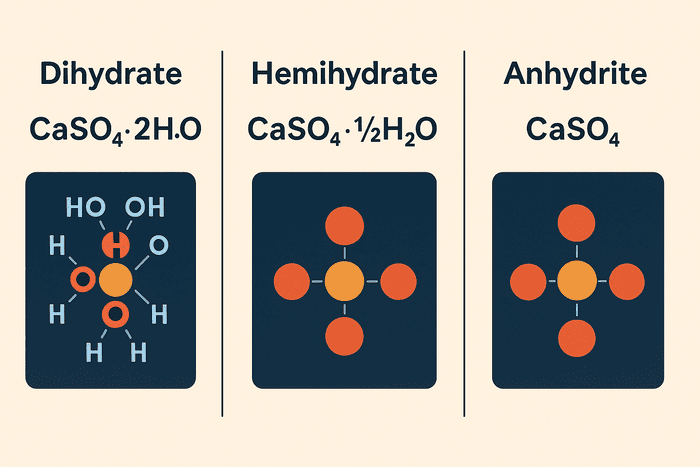
Calcium sulfate is one of the most widely used mineral compounds in the construction and industrial world. While many people recognize its role in the production of plaster, drywall, and cement, fewer realize that calcium sulfate exists in three distinct forms—each with its own structure, properties, and applications. These forms are: dihydrate (CaSO₄·2H₂O), hemihydrate (CaSO₄·½H₂O), and anhydrite (CaSO₄).
In this article, we’ll take a closer look at what sets these forms apart, how they are produced, and why each one is uniquely valuable in specific industrial and commercial uses. Whether you’re an engineer, builder, or just a curious reader, understanding these differences can help you make better choices in both design and material selection.
Understanding the Chemistry: What Do These Forms Mean?
The names dihydrate, hemihydrate, and anhydrite refer to the amount of water chemically bound within the calcium sulfate structure. This water isn’t just physically mixed with the mineral—it’s part of the crystalline form. That means heating or hydrating calcium sulfate can actually change it from one form to another.
- Dihydrate is the most naturally occurring form, commonly known as gypsum.
- Hemihydrate is what we know as plaster of Paris.
- Anhydrite is the completely water-free version of calcium sulfate.
These differences might seem small, but they drastically affect how the material behaves when heated, mixed with water, or used in construction.
Gypsum (Calcium Sulfate Dihydrate): The Natural Form
Gypsum (CaSO₄·2H₂O) is a naturally occurring mineral that contains two water molecules for every molecule of calcium sulfate. It is soft, white to grayish, and widely found in sedimentary rock formations around the world.
Gypsum’s most notable feature is its solubility and reactivity with heat. When heated to around 150°C (300°F), it releases part of its water and transforms into hemihydrate—this is the foundation of plaster manufacturing. Gypsum is extensively used in agriculture as a soil conditioner and in construction as a base material for plaster, drywall (gypsum boards), and cement.
Because it is relatively soft and easy to work with, gypsum is ideal for interior wall applications. Its ability to help regulate indoor humidity by absorbing and releasing moisture adds another layer of appeal for architects and designers.
Plaster of Paris (Calcium Sulfate Hemihydrate): Quick-Setting and Versatile
When gypsum is heated, it loses about 75% of its water content and becomes calcium sulfate hemihydrate (CaSO₄·½H₂O), commonly known as plaster of Paris. This material is highly reactive with water, and when it is mixed back with water, it rehydrates and quickly hardens into its original dihydrate form.
This rapid setting time makes plaster of Paris perfect for molding, casting, and decorative applications. It is widely used in sculpting, medical orthopedics (like casts), false ceilings, and ornate architectural elements. Its fine texture and ability to capture detail make it the preferred material for intricate designs.
However, this quick setting can also be a downside for large-scale applications, requiring skilled labor and proper timing. To improve workability, retarding agents are often added to extend the open time.
Anhydrite (Calcium Sulfate Anhydrous): The Industrial Heavyweight
Anhydrite is the anhydrous form of calcium sulfate (CaSO₄) and contains no water molecules in its crystal structure. It is usually found deeper underground than gypsum and is harder and denser in comparison. Because of its stability and low reactivity with water, anhydrite is used in applications that require high strength and long-term durability.
Unlike gypsum and hemihydrate, anhydrite doesn’t rehydrate easily, which means it has to be chemically activated for certain applications. It’s commonly used in:
- Self-leveling floor screeds
- Dry mortars and tile adhesives
- Industrial drying agents
- Cement additives for controlling setting time
Due to its slow setting behavior, anhydrite allows for longer working times, which is especially beneficial in large-scale flooring or construction projects.
Key Differences at a Glance
While all three forms share the same chemical base (CaSO₄), their water content and crystal structure change everything:
- Gypsum is soft, rehydratable, and naturally abundant.
- Hemihydrate is quick-setting, ideal for casting and finishing.
- Anhydrite is stable, hard, and suited for industrial use where durability is key.
Their thermal behavior is another key differentiator. Gypsum becomes hemihydrate upon heating. Hemihydrate can revert to gypsum with water. Anhydrite, however, requires higher temperatures to form and doesn’t easily return to a hydrated state without additives.
Practical Implications for Industry and Construction
Choosing the right form of calcium sulfate isn’t just about availability—it’s about the needs of the project. If speed and decorative detail are priorities, hemihydrate is your best bet. If long-term strength and moisture resistance are required, anhydrite takes the lead. And for general-purpose use, gypsum offers an affordable, sustainable solution.
This is especially important in regions with specific climate or building regulations. For instance, gypsum’s fire-resistant properties make it ideal for partition walls in high-rise buildings, while anhydrite’s mechanical strength is better suited for industrial flooring systems.
Conclusion: One Material, Many Possibilities
Calcium sulfate may appear to be a simple compound, but its three forms—dihydrate, hemihydrate, and anhydrite—offer an impressive range of physical and chemical characteristics. Whether you’re constructing buildings, designing interiors, or manufacturing industrial products, understanding the subtle differences between these forms empowers you to choose the right material for the right job.
At a time when efficiency, sustainability, and performance matter more than ever, calcium sulfate in its various forms remains a trusted and versatile solution across industries.






