
Is Gypsum Metallic or Nonmetallic?
Gypsum is a widely recognized mineral that plays a crucial role in industries such as construction, agriculture, and medicine. However, when classifying minerals, one of the fundamental distinctions is between metallic and nonmetallic minerals. So, where does gypsum fit in? Gypsum is a nonmetallic mineral. This classification is based on its physical and chemical properties, as well as its industrial applications.
In this article, we will explore the characteristics of gypsum, why it is considered nonmetallic, how it differs from metallic minerals, and its numerous uses in various industries.
Understanding Gypsum: Composition and Formation
Gypsum is a calcium sulfate dihydrate mineral, with the chemical formula CaSO₄·2H₂O. This means it is composed of calcium, sulfur, oxygen, and water molecules. It is primarily formed through the evaporation of saline water bodies such as lakes and seas. Over time, as the water evaporates, minerals like gypsum crystallize and accumulate in sedimentary deposits.
Gypsum is commonly found in arid regions where ancient seas once existed, and it often occurs alongside other evaporite minerals such as halite (rock salt) and anhydrite. Some of the world’s largest gypsum deposits are found in North America, Europe, and the Middle East.
Why is Gypsum Considered a Nonmetallic Mineral?
Minerals are broadly classified into two categories:
Metallic minerals – These contain metal elements in their composition and often have properties such as high conductivity, malleability, and metallic luster. Examples include iron, copper, and gold.
Nonmetallic minerals – These do not contain metal elements in significant amounts and generally lack the characteristics of metallic minerals. Examples include quartz, feldspar, and gypsum.
Key Properties of Gypsum that Make It Nonmetallic
Several distinct properties of gypsum set it apart as a nonmetallic mineral:
Luster and Appearance – Metallic minerals typically exhibit a shiny, reflective surface, also known as metallic luster. In contrast, gypsum has a dull to vitreous (glassy) luster and does not reflect light like metals do. Some varieties, like selenite, may appear slightly translucent, but they still lack a metallic sheen.
Conductivity – One of the key features of metallic minerals is their ability to conduct electricity. Gypsum, on the other hand, is an insulator and does not conduct electricity, further confirming its nonmetallic nature.
Hardness – Gypsum is a relatively soft mineral, ranking 2 on the Mohs hardness scale. This means it can be easily scratched by a fingernail. Metallic minerals tend to be much harder and more resistant to scratches. For example, iron has a Mohs hardness of 4-5, while quartz (a nonmetallic mineral) ranks at 7.
Cleavage and Fracture – Gypsum has perfect cleavage in one direction, meaning it can split into thin, flat sheets. This characteristic is commonly seen in nonmetallic minerals. Metallic minerals, on the other hand, often exhibit irregular fracture patterns rather than distinct cleavage planes.
Solubility in Water – Gypsum is water-soluble, meaning it dissolves in water over time. This is quite rare for metallic minerals, which are usually resistant to dissolution. This property makes gypsum particularly useful in soil treatment, as it helps improve soil structure when it dissolves.
Reaction with Acid – While carbonate minerals (such as limestone) react with acids by producing bubbles of carbon dioxide, gypsum does not react in the same way. This behavior is another indication of its nonmetallic classification.
How Gypsum Differs from Metallic Minerals
To further emphasize the distinction between gypsum and metallic minerals, let’s compare gypsum to some common metallic minerals:
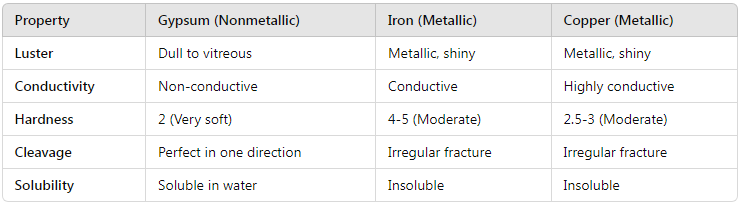
From this comparison, it is clear that gypsum lacks the defining characteristics of metallic minerals, reinforcing its classification as nonmetallic.
Industrial Applications of Gypsum
Because gypsum is a nonmetallic mineral, it is used in a variety of industries that require materials with insulating, soft, and water-soluble properties. Some of the most common applications include:
- Construction Industry
Gypsum is a major component in drywall (plasterboard or sheetrock), which is used to construct interior walls and ceilings.
It is used in cement production to regulate the setting time of concrete.
Gypsum is also an ingredient in plaster of Paris, which is used for making molds, sculptures, and decorative wall finishes.
- Agriculture
Gypsum is used as a soil conditioner to improve soil structure, prevent compaction, and enhance water infiltration.
It helps neutralize saline soils, making them more suitable for agriculture.
Farmers use gypsum to supply essential nutrients like calcium and sulfur to crops.

- Medicine and Orthopedics
Gypsum-based plaster of Paris is used for making orthopedic casts to immobilize broken bones.
It is used in dental molds to create accurate impressions of teeth.
- Art and Sculpture
Artists use gypsum-based plasters to create intricate sculptures and moldings.
It is a key material in decorative ceiling and wall designs.
- Environmental Applications
Gypsum helps in wastewater treatment by removing impurities.
It is used in reducing sulfur dioxide emissions in industrial processes, making it an eco-friendly material.
Conclusion: Gypsum is a Nonmetallic Mineral
To summarize, gypsum is nonmetallic because it lacks the properties associated with metallic minerals, such as conductivity, metallic luster, and hardness. Instead, it is soft, water-soluble, and has perfect cleavage, making it valuable in various industries, particularly construction and agriculture.
Understanding the classification of minerals like gypsum helps industries and researchers make informed decisions about their applications. Whether in building materials, farming, or medicine, gypsum remains a versatile and essential nonmetallic mineral.







